


Note that many of the pages are not up yet, so please show patience with links leading to blank pages and dead ends.
Currently the followin journals are included:
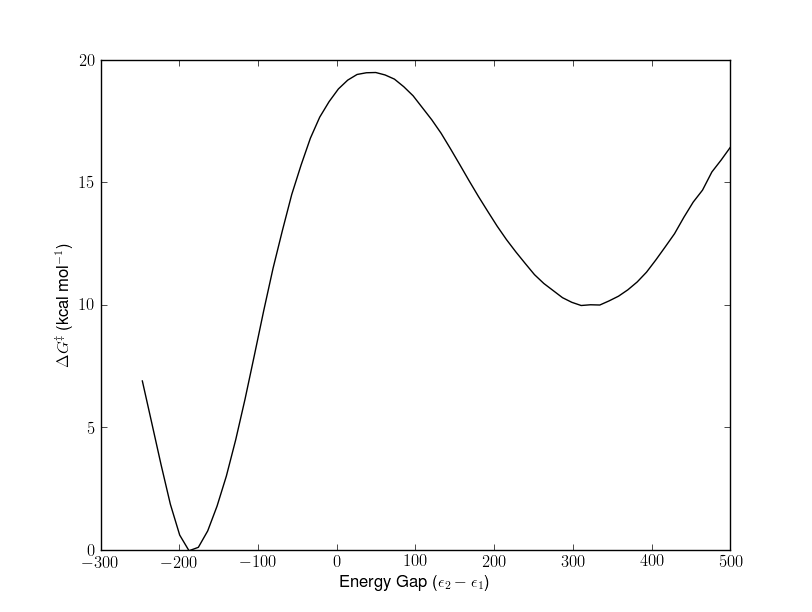
In a currently ongoing project I have calculated the rate limiting step in the acylation reaction, the formation of the tetrahedral intermediate, using the MD/FEP/EVB technique. The activation energy (transition state, TS), Δ G‡ , obtained from the calculations are around 19.5 kcal/mol. This is in excellent agreement with the substrate dependent barrier that typically is 15 - 20 kcal/mol.
The reaction profile is illustrated below where RS is the reactant state, TS the transition state and TET is the tetrahedral intermediate.
A animation with 100 ps of the trajectory around the transition state illustrates the system changing from the reactant state to the tetrahedral intermediate. Notice that water molecules (~5000) have been hidden in the animation for better visibility. Only the counter ions (Cl-) are visible.
View the animation here!